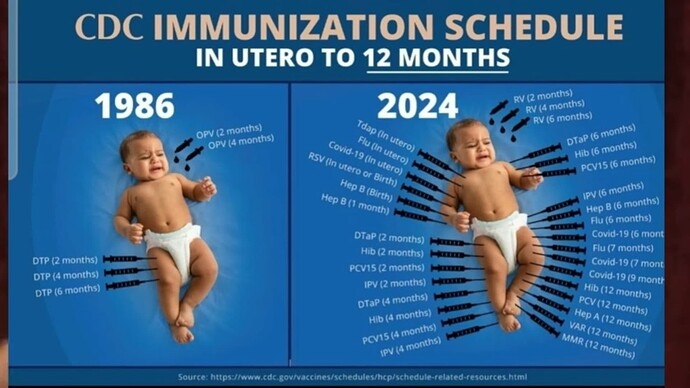
CDC Adds COVID Jab to Childhood Immunization Schedule
STORY AT-A-GLANCE
Unlicensed COVID-19 shots will be added to the U.S. childhood, adolescent and adult vaccine schedules after a unanimous (15-0) vote by the U.S. CDC’s Advisory Committee on Immunization Practices
By adding the shots to the vaccine schedule, it paves the way for U.S. schools to mandate them for attendance
-
Pfizer and Moderna, the shots’ makers, will also be granted permanent legal indemnity, which otherwise would have disappeared once COVID-19 shots were no longer protected under emergency use authorization
-
Once the CDC recommends a shot for “routine administration to children or pregnant women,” it becomes liability free
-
Even though COVID-19 shots have been added to the childhood vaccine schedule, they haven’t been mandated for school attendance in most states — yet. In the event that they are — and even before that — it’s time for dissent and a complete overhaul of the CDC
https://articles.mercola.com/sites/articles/archive/2022/10/28/kid-covid-shots-immunization-schedule.aspx
ACIP Membership Roster
Advisory Committee on Immunization Practices (ACIP)
Advisory Committee on Immunization Practices (ACIP) Home
(https://www.cdc.gov/vaccines/acip/members/bios.html#)
COMMITTEE MEMBERS
ACIP Membership Roster
Advisory Committee on Immunization Practices (ACIP)
Advisory Committee on Immunization Practices (ACIP) Home
ACIP Membership Roster
Lynn Bahta, RN, MPH, CPH

Immunization Program Clinical Consultant, Minnesota Department of Health, Saint Paul, Minnesota. Term: 7/1/2019 – 6/30/2023
Ms. Bahta serves as the clinical expert for vaccines at the Minnesota Department of Health. She has 25 years of experience in the field of immunization, including both adult and pediatric clinical nursing and in local and state public health.
Beth P. Bell, MD, MPH

Clinical Professor, Department of Global Health, School of Public Health, University of Washington, Seattle, Washington. Term: 7/1/2019 – 6/30/2023
Dr. Bell is a Clinical Professor in the Department of Global Health at the University of Washington School of Public Health where she leads efforts to improve work in the areas of pandemic preparedness and global health security. She spent most of her career in government service at CDC; she served as the Director of the National Center for Emerging and Zoonotic Infectious Diseases at CDC, until her retirement in January 2017.
Oliver Brooks, MD, FAAP

Oliver Brooks, MD, FAAP, Chief Medical Officer, Watts HealthCare Corporation, Los Angeles, CA. Term: 7/26/2021 – 6/30/2025
Dr. Brooks is Chief Medical Officer at Watts Healthcare Corporation in Los Angeles, California. He is a past president of the National Medical Association (NMA), and past president of the California Immunization Coalition. He was co-chair of the California COVID-19 Vaccine Work Group, which determined allocation of the COVID-19 vaccine in the state, and had worked
for more than 15 years in various leadership roles focusing on disparities in vaccine coverage rates.
Wilbur H. Chen, MD, MS, FIDSA, FACP
Dr. Chen is a Professor of Medicine at the University of Maryland School of Medicine and Director of the University of Maryland Baltimore Travel Medicine Practice. He has a specific research interest in developing vaccines against pathogens which afflict low- and middle-income countries and is a co-investigator of the NIAID-funded Vaccine Treatment and Evaluation Unit and NIAID-funded Collaborative Influenza Vaccine Innovation Centers.
Sybil Cineas, MD, FAAP, FACP

Sybil Cineas, MD, FAAP, FACP, Associate Professor of Medicine, Pediatrics, and Medical Science (Clinical), The Warren Alpert Medical School of Brown University, Associate Program Director, Brown Combined Residency in Internal Medicine and Pediatrics, Providence, RI. Term: 7/28/2021 – 6/30/2025
Dr. Cineas is dual board certified in Internal Medicine and Pediatrics and serves as the Associate Program Director for the Brown Combined Internal Medicine-Pediatrics Residency Program. In addition to servings as a primary care provider for patients of all ages, she is highly involved in the training of residents and medical students. She has 20+ years of experience teaching about and promoting vaccination in the clinical setting.
Matthew F. Daley, MD

Senior Investigator, Institute for Health Research, Kaiser Permanente Colorado, Aurora, Colorado. Term: 1/4/2021 – 6/30/2024
Dr. Daley is a practicing pediatrician with extensive research experience in the areas of vaccine safety, parental vaccine hesitancy, and immunization services delivery. Dr. Daley is an investigator in the Vaccine Safety Datalink (VSD), leading or contributing to studies of the safety of newly licensed vaccines, the safety of vaccines during pregnancy, and the safety of the immunization schedule. Dr. Daley is also an Associate Professor in the Department of Pediatrics at the University of Colorado School of Medicine.
Camille N. Kotton, MD, FIDSA, FAST

Clinical Director, Transplant and Immunocompromised Host Infectious Diseases, Infectious Diseases Division, Massachusetts General Hospital and Associate Professor, Harvard Medical School, Boston, Massachusetts. Term: 12/23/2020 – 6/30/2024
Dr. Kotton is an infectious disease clinician with special expertise in the care of complex patients undergoing solid organ transplantation and with cancer. She is a national expert in vaccination and zoonotic infectious diseases in the immunocompromised host. Dr. Kotton’s clinical publications include major reviews on zoonotic infections, travel vaccines, and viral infections after transplantation.
Jamie Loehr, MD, FAAFP

Jamie Loehr, MD, FAAFP, Owner, Cayuga Family Medicine, Ithaca, NY. Term: 7/26/2021 – 6/30/2025
Dr. Loehr has practiced as a family physician in Rochester and Ithaca, NY, for over 30 years, counseling patients every day on the benefits of vaccines. He served as the AAFP liaison to the ACIP for four years and has been a member of the ACIP influenza working group for over 10 years.
Grace M. Lee, MD, MPH

Associate Chief Medical Officer for Practice Innovation, Stanford Children’s Health and Professor of Pediatrics, Stanford University School of Medicine, Stanford, California. Term: 8/4/2021 – 6/30/2023
Dr. Lee is board certified in pediatric infectious diseases and currently serves as Associate Chief Medical Officer for the health system focused on bridging quality, research and implementation. She previously served as the Principal Investigator on the CDC-funded Vaccine Safety Datalink project, Associate Director of the FDA-funded Mini-Sentinel Project, and a Board Member for the National Academy of Medicine Board on Population Health and Public Practice.
Sarah S. Long, MD

Professor of Pediatrics, Drexel University College of Medicine and Attending Physician, Infectious Diseases, St. Christopher’s Hospital for Children, Philadelphia, Pennsylvania. Term: 12/24/2020 – 6/30/2024
Dr. Long is board certified in pediatrics and pediatric infectious diseases and has over four decades of contributions to the field of pediatric infectious disease. She has served on numerous committees including as a standing member on the Vaccine and Related Biological Products Advisory Committee (VRBPAC) of the FDA and as a member of the American Academy of Pediatrics (AAP) Committee on Infectious Diseases.
Veronica V. McNally, J.D.

President and CEO, Franny Strong Foundation, East Lansing, Michigan. Term: 10/31/2018 – 6/30/2023
Ms. McNally is the President and CEO of the Franny Strong Foundation. This foundation was created in memory of her daughter, who lost her life to pertussis, to promote vaccinations and education about vaccinations. Ms. McNally has done research on vaccines since the loss of her child and has made it her mission to ensure all parents have good information available to make informed decisions about vaccines.
Katherine A. Poehling, MD, MPH

Professor of Pediatrics and Epidemiology and Prevention, Wake Forest School of Medicine, Winston-Salem, North Carolina. Term: 7/1/2019 – 6/30/2023
Dr. Poehling is board certified in pediatrics and an expert on the community impact of vaccines, specifically pneumococcal and influenza vaccines. She serves as the NC American Academy of Pediatrics Immunization Representative and has collaborated with CDC on the New Vaccine Surveillance Network and with the CDC Active Bacterial Core Surveillance group.
Pablo J. Sanchez, M.D.

Professor of Pediatrics, Division of Neonatal-Perinatal Medicine, Division of Pediatric Infectious Diseases, The Ohio State University – Nationwide Children’s Hospital, Columbus, Ohio. Term: 7/1/2019 – 6/30/2023
Dr. Sanchez is board certified in both neonatology and pediatric infectious diseases. He has had a distinguished career as a clinical and scientific investigator in neonatal and perinatal infections with major contributions in areas such as congenital syphilis, congenital cytomegalovirus (CMV), respiratory syncytial virus (RSV) infections, neonatal sepsis, antimicrobial stewardship in the Neonatal Intensive Care Unit (NICU), and immunizations in premature infants.
Nirav D. Shah, MD, JD

Director, Maine Center for Disease Control and Prevention, Augusta, ME. Term: 9/26/2022 – 6/30/2026
Nirav Shah is the Director of the State of Maine Center for Disease Control and Prevention. Shah previously served as the Director of the Illinois Department of Public Health. Earlier in his career, he worked for the Cambodian Ministry of Health. He also served as President of the Association of State and Territorial Health Officials (ASTHO).
Helen Keipp Talbot, MD, MPH

Associate Professor of Medicine, Division of Infectious Diseases, and Associate Professor of Health Policy, Vanderbilt University, Nashville, Tennessee. Term: 10/29/2018 – 6/30/2023
Dr. Talbot is an internist and infectious disease specialist at Vanderbilt University. Her research on immunization spans the age spectrum from pediatric to adults, with a special focus on immunization issues in older adults. Dr. Talbot is highly accomplished in assessing the impact of influenza vaccines on disease burden and is a leader in the study of vaccines in older adults.
https://www.cdc.gov/vaccines/acip/index.html
https://www.cdc.gov/vaccines/acip/members/index.html
https://www.cdc.gov/vaccines/acip/members/members-archive.html