The Low-Down on PCR and Lateral Flow Tests
We're only going to look at three companies, with Gibraltar's AllTest being last up:
1) Innova Medical Group lateral flow test for Covid-19 had been declared unfit for purpose, yet the ![]() UK still bought them – The Innova scandal Part 4: Questions the government must answer - The Conservative Woman
UK still bought them – The Innova scandal Part 4: Questions the government must answer - The Conservative Woman
2) FlowFlex –
You may recognize this image as it was featured in the ![]() Gibraltar Chronicle – GSD says Govt should allow sale of low-cost LFTs. The use of Flowflex might have been strategic because this so-called "cheap" version has a rap-sheet, or at least the version in the blue box does:
Gibraltar Chronicle – GSD says Govt should allow sale of low-cost LFTs. The use of Flowflex might have been strategic because this so-called "cheap" version has a rap-sheet, or at least the version in the blue box does:

Articles that explain the difference:
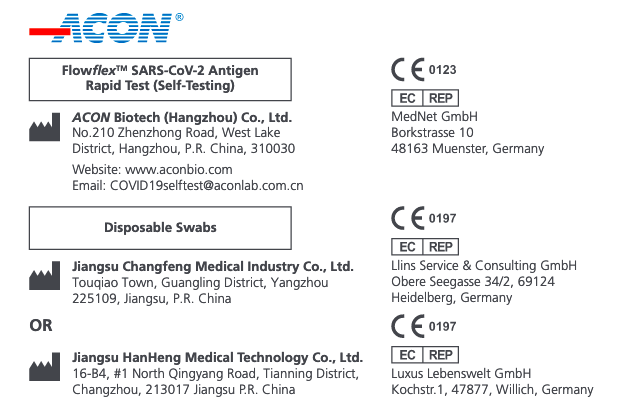
And of course, it's made in China. Interesting choice of names - A CON
ACON Flowflex is used by the NHS – From the UKGOV: Your step-by-step guide for ACON Flowflex™ SARS-CoV-2 Antigen Rapid Test (Self-Testing).
Here is a list of the ones approved by the UK – COVID-19 test approval: how to apply - GOV.UK
AllTest doesn't seem to be on the list above (currently), but it has been approved – Lateral Flow Rapid Antigen Test Kits | ADTUK – ADTUK Wholesale Medical Supplies
3) AllTest – ![]() Gibraltar's Test.
Gibraltar's Test.
Our rapid test is a nasopharynx (nasal swab) lateral flow diffusion antigen test (ALLTEST SARS-CoV-2 Antigen Rapid Test). The lateral flow / diffusion rapid test gives a result in less than an hour, because they don't have to be sent to a lab to be processed. Does your rapid test meet UK Government requirements for entry to the UK from Gibraltar? Yes, our rapid test meets UK Government guidelines for international travellers entering the UK from Gibraltar – Rapid Covid Tests At Gibraltar Airport – About our tests
It's been approved by Spain and the EU, as outlined by these two articles:
Unapproved Hangzhou products were being bought by the US and UK:
Unapproved Chinese coronavirus antibody tests being used in at least 2 states
Two U.S. companies — Premier Biotech of Minneapolis and Aytu Bioscience of Colorado — have been distributing the tests from unapproved Chinese manufacturers, according to health officials, FDA filings and a spokesman for one of the Chinese manufacturers. Tests made by two Chinese manufacturers, Hangzhou Biotest Biotech Co. and Zhejiang Orient Gene Biotech, are not on the approved list, but they are being sold in the U.S.
Faulty masks. Flawed tests. China's quality control problem in leading global COVID-19 fight
Britain reportedly bought 3.5 million antibody tests from two Chinese companies, Guangzhou Wondfo Biotech Co. and Hangzhou AllTest Biotech Co. Only one of those, Wondfo, was on the Chinese National Medical Products Administration’s list of authorized test kits.
Georgia has canceled a contract with the Chinese company that sent flawed test kits to Spain, and Malaysia has opted to buy testing kits from South Korea instead of China because of the Chinese tests’ reported low accuracy rate.
Even Chinese don’t trust ’em: FDA urged to recall, ban imports of rapid test kits rejected in China
They've cleaned up their ACT because now AllTest is just fine. And since so-called scientists reviewed them, governments can push them:
- Alltest rapid lateral flow immunoassays is reliable in diagnosing SARS-CoV-2 infection from 14 days after symptom onset: A prospective single-center study - PubMed
- Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2 - PubMed
TADA.
Yes, they can make this stuff up as they go along, correcting their science, making false claims such as how effective all their measures are.
And as long as everyone keeps buying what they are selling, there will be no end:
Proverbs 27:20 Hell and destruction are never full; so the eyes of man are never satisfied.